In part 1 of this series, I shared a couple of images of a DMT Fine (600) diamond plate before and after “break-in.”
In this article, I present a few images from a Coarse (325) and an Extra-Fine (1200) diamond plate. These images provide insight into the surprising results of The Diamond Plate Progression.
The Coarse plate is very similar to the Fine, with a relatively uniform size distribution of diamond particles embedded in a nickel plating.

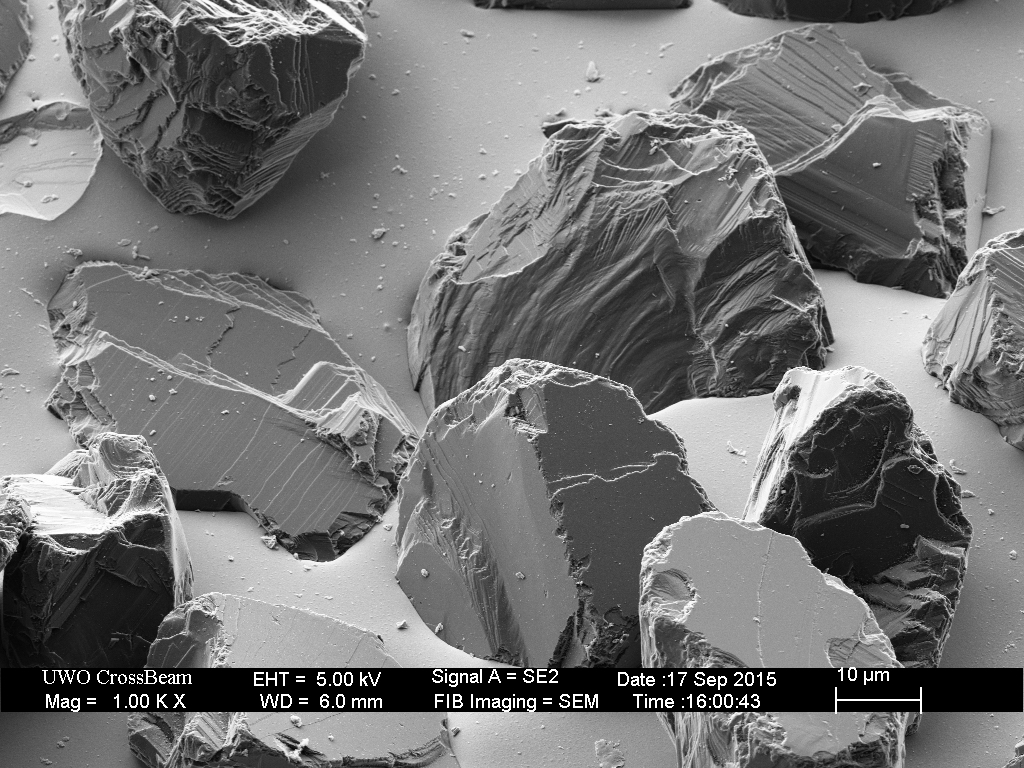
In this example, the hone was “broken-in” by rubbing on a hardened steel surface for approximately ten minutes and then used to lap a Shapton 16k hone for a few minutes. The results are consistent with those observed in the case of the Fine hone. The diamonds that sit higher are removed or broken off, evening out the variation in the height of the exposed diamonds and smoothing the diamonds that contact the surface being honed.


The Extra-Fine (1200 grit) is of similar construction, with appropriate-sized diamonds embedded in nickel plating.

Surprisingly, this plate is “contaminated” with over-sized diamond particles. These coarse-sized diamonds are approximately twenty microns higher than the nominal-sized (9 micron) diamonds. Such over-sized particles are likely responsible for the anomalously deep scratches created by the EF and EEF hones in The Diamond Plate Progression.

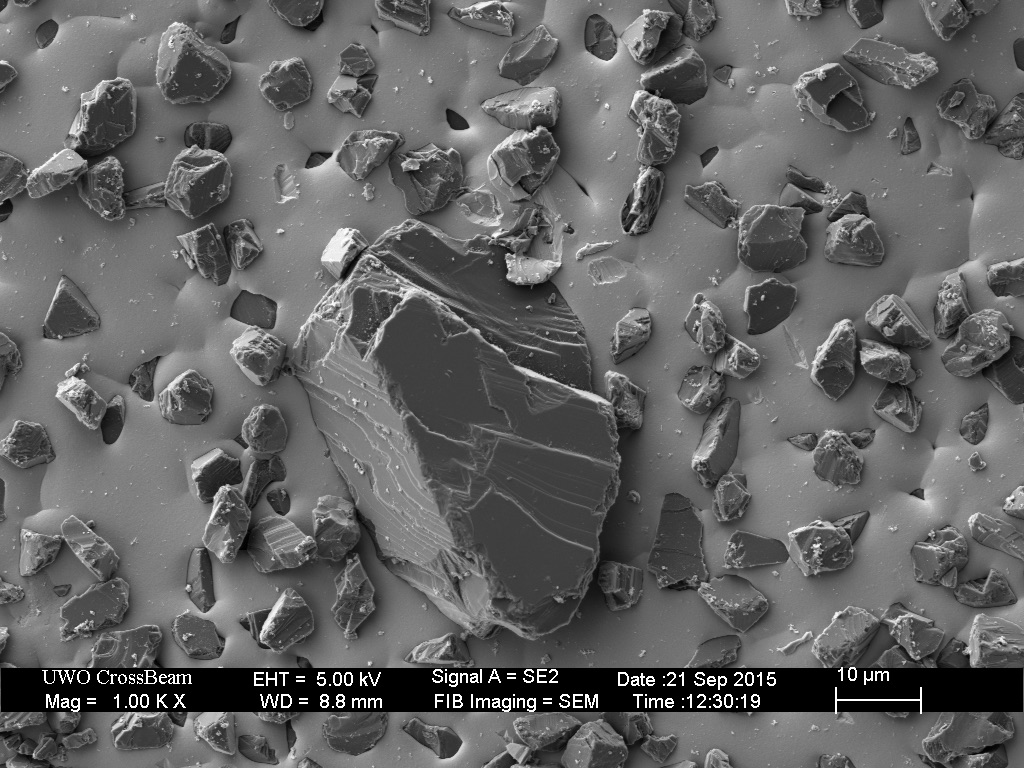

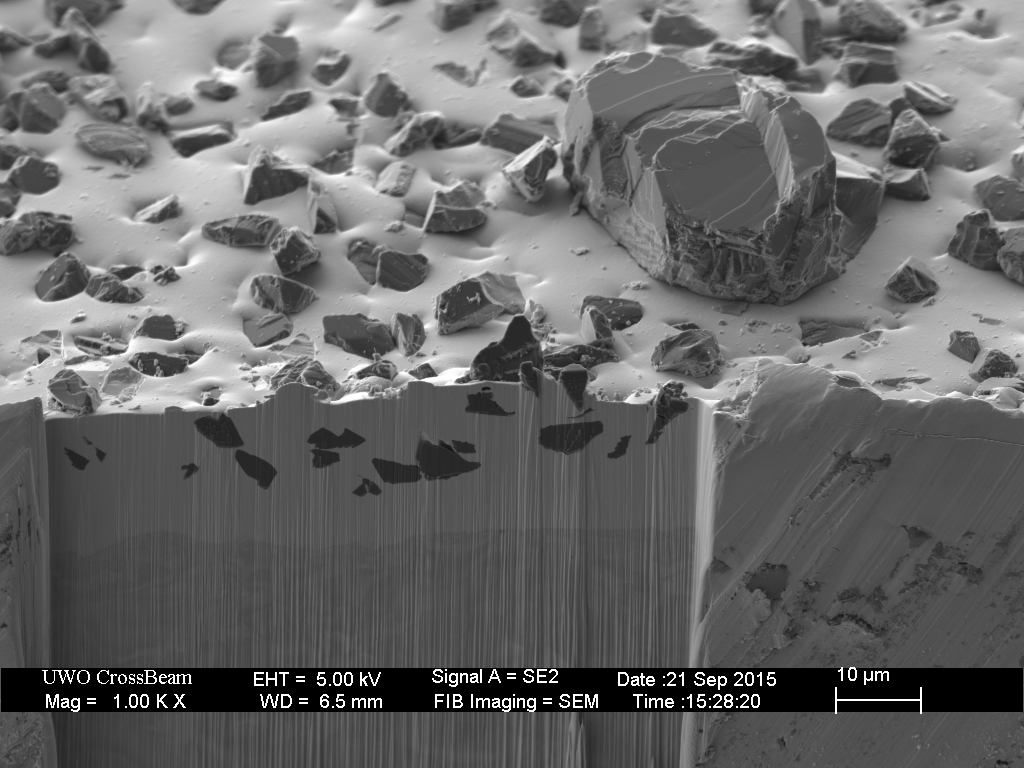
This plate was then broken-in with the usual process of rubbing a piece of hardened steel across the surface for approximately ten minutes.

Closer inspection of the remaining over-sized diamonds reveals several outcomes.
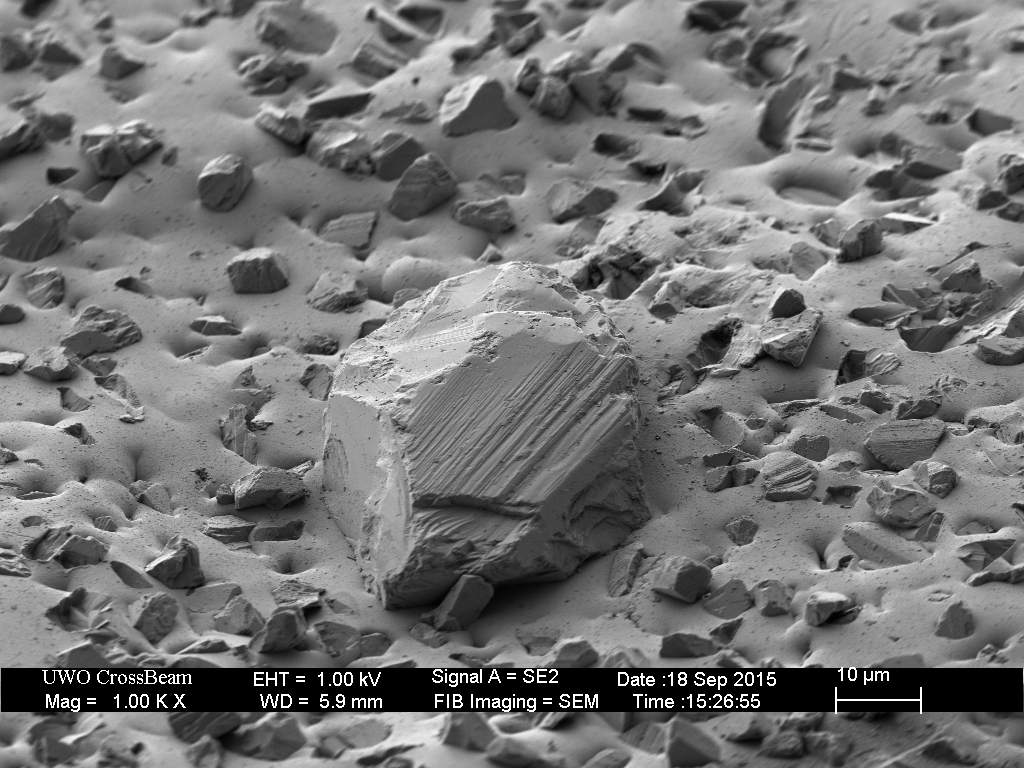
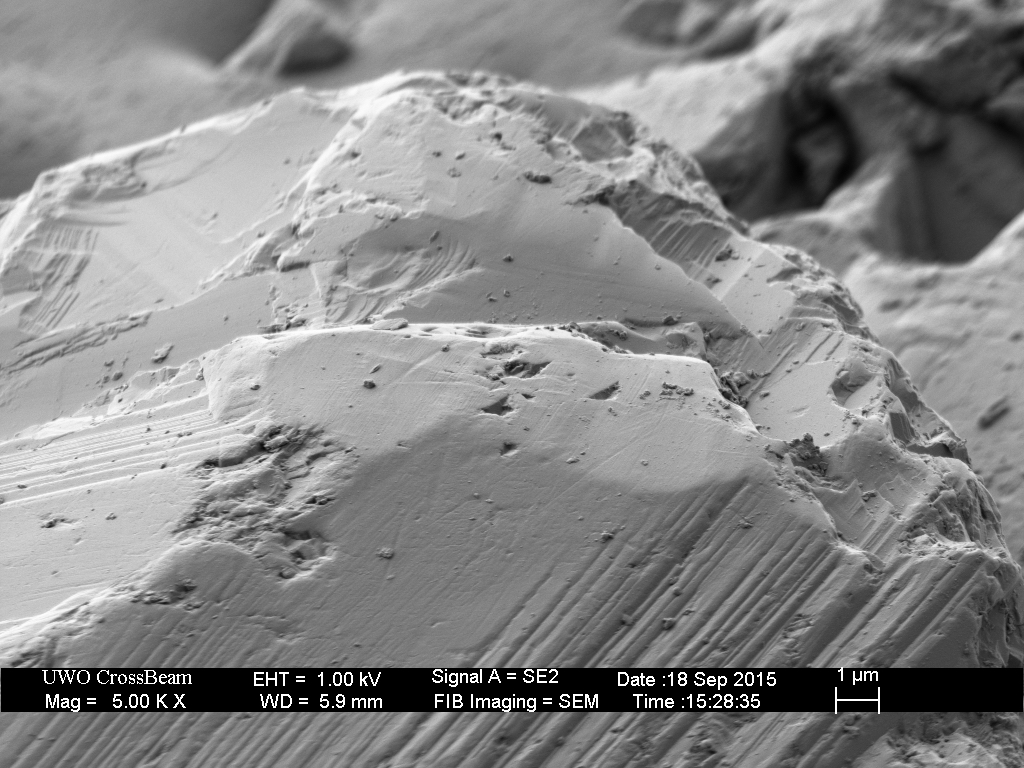

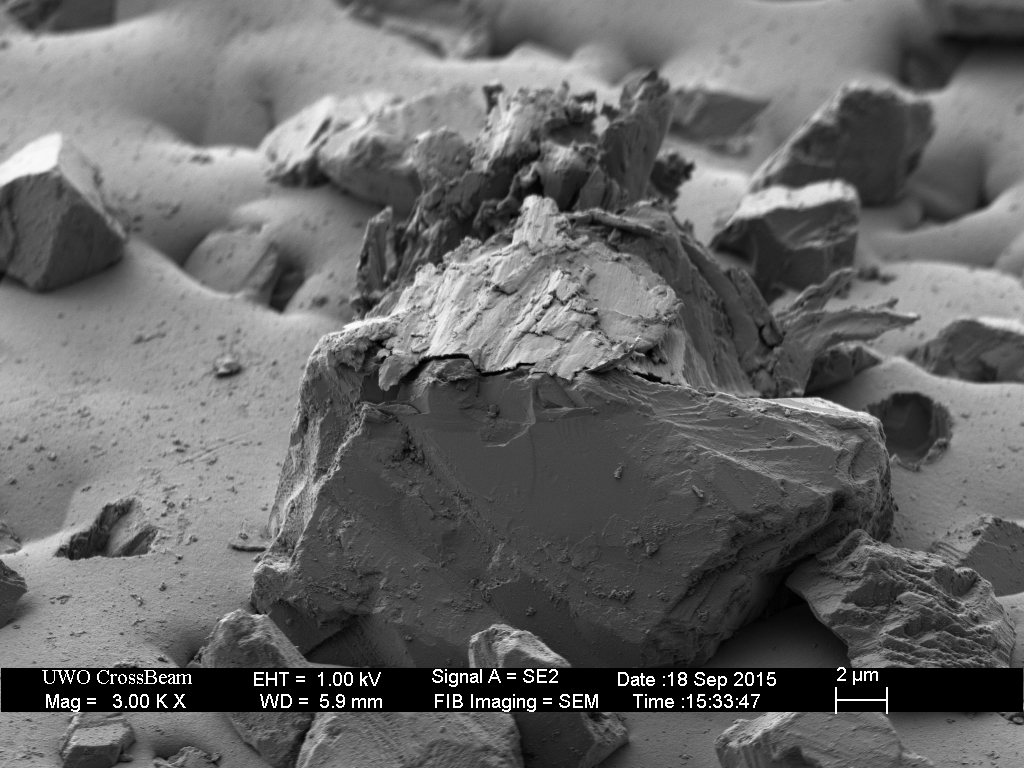
The break-in procedure affects the extra-fine (9 micron) particles as well. The less well adhered diamonds fall out, and many are broken.


The before and after images presented here demonstrate that significant changes occur in the surface of the plates occur within the first few minutes of use. The depth of scratches created by these hones will depend on the shape of the exposed tips and on the applied pressure.
Noting that pressure is inversely proportional to contact area, when honing on the extra fine plate with its few tall coarse diamonds, the pressure will be very high with the force spread over those few contact points, resulting in anomalously deep scratches. Successful break-in will require complete removal of the over-sized diamonds. The challenge is to find a break-in procedure that will remove the over-sized particles while minimizing the loss of nominal-sized abrasive.

26 responses to “Diamond Plate break-in part 2”
I keep thinking higher impact speed must be proportional to better cleavage of diamond high parts as in the macro world of, say, flintknapping by percussion vs pressure flaking, or a diamond cutter striking to split the diamond. In my imagination a longer, slower contact time with the steel would be more likely to either do nothing or rip a diamond out of its bed. Likewise a rapid impact (impulse in physics) should be more likely to fracture the diamond at the part that is sticking up. I received a new DMT 325 last week and for break in I used the flat of an old chisel whipped back and forth with weight of the chisel only being the downward pressure. My current highest magnification is 120x so I don’t know the results, however a Naniwa 12k was very smooth after lapping…
LikeLike
I suppose if the goal is to fracture the diamonds rather than smooth them, we could try using it dry rather than with lubrication.
LikeLike
A simple larger scale but related experiment- hold a flint in one hand (barely) gripping with thumb and pointer only, and a hammer stone gripped with full fist in the other hand. If the hammer stone is slowly pushed onto the flint with increasing pressure the two finger grip will fail and drop the flint. However, if the hammer stone rapidly strikes the edge of the flint a flake will be knocked off leaving the main part of the flint in the two finger grip.
LikeLike
Is it possible the top of the coarse diamond in image #s 11, 12 from the top is not ‘smoothed’, but was that way before break in ? The top of the one in #4 looks like many tiny fractures.
Also, having a small DMT 1200 in my briefcase I couldn’t wait to look at it 100x. This one is a couple years old and looks very much like img# 10. The depressions where coarse were are spaced similarly, and after some searching I was able to locate a coarse still attached ! I have a newer one at home, this eve I plan to check it too. I will attempt to mark near some attached coarse with a sharpy, then try a few high speed low pressure laps with the somewhat square back of an old hickory butcher knife, then note if I see a depression or not. If not, that could indicate it broke rather than ripped out.
Thanks so much for your effort. I’m sure many of the other razor guys will be checking their DMT 1200s for boulders since so many of us have 100x optical. I have looked at its surface before just out of curiosity but didn’t know what to look for till seeing your work. thanks again.
LikeLike
Thanks for that. It makes me wonder whether these over-sized diamonds are deliberately added to enhance wear resistance.
None of the over-sized diamonds in the as-received plate that I looked at (about 20) had a smooth area on the top surface. I took images from a few of those as “before” shots, but all of them were lost during break-in. I did not image that particular diamond before, so I can’t be sure.
LikeLike
A second, newer DMT 1200 has the same condition. Having had it a couple months I (mistakenly) considered it broken in after the usual initial rubbing routine. After scrubbing the surface with comet I examined it with a pocket microscope, Carson Microbrite 60-120x. This scope has a base allowing it to rest on the DMT and remain in focus while moving over the surface. While some regions are free of coarse diamonds enough to mislead with just a quick look, a harder look revealed many. Locating a cluster of these while at 120x, I was able to mark around them with tip of a 0.7mm mech pencil that showed up surprisingly well in the LED light of the scope. I also outlined the base of the scope to ease finding the spot again. The mark around the cluster was invisible to my naked eye and barely visible with a 3x hand glass. I state this to give some perspective to the closeness of a cluster of 4 coarse. I had to zoom out to 60x to find the spot again and was surprised to be able to still see the coarse group. While watching at 60x I used the point of a small knife to scrub the cluster. Shiny steel from the knife marked the DMT almost as well as the pencil while scrubbing, yet the course stayed in the DMT. Perhaps some others will try this. A sad revelation- boulders on the runway.
LikeLike
It is interesting to see the wear on the plates that you used, both on the diamonds themselves and on the nickel in which they are imbedded. Have you ever tried doing the same experiment using lapping films or a product like Micro-Mesh? The description of Micro-Mesh MXD sheets says that the diamonds are held in a flexible resin layer that should reduce or eliminate the effect of having some particles stand higher than others, and should therefore eliminate the anomalously deep scratches you mentioned and produce a finer scratch pattern and more linear edge. I would imagine too that a flexible resin matrix would reduce the number of diamonds that are broken or torn out, and that there would be no need for it to be “broken in” like a diamond plate.
LikeLike
Lapping film and micro-mesh work by the same principle as a strop – the resiliency of the material allows larger particles (which sit higher, make first contact, and experience higher pressure) to be pushed down into the substrate, preventing them from producing deep scratches. Another way to think of this is that the substrate allows pressure to equalize across all grit particles by allowing the ones that see higher pressure to sink down into the substrate.
The one (potential) negative of those substrates is that they result in a slight convexity to the bevel – increasing the near-apex angle beyond the sharpening angle. This effect is mitigated by increasing the sharpening angle with subsequent steps, or by continuing the progression with resilient substrates at the same angle.
“Break-in” on those substrates is desirable, in that we want to remove the loosely bonded grit particles. The particles are not bonded firmly enough to be “broken” as we observe in the nickel/diamond plate.
LikeLike
Hi Todd,
First, a special word of appreciation for your excellent website and the very informative work presented.
In 2009 I bought a couple of DMT EEF (#8000) plates, broke them in by lapping against a Spyderco UF ceramic plate, and was very impressed with their performance on both knives and woodworking tools. So impressed in fact that in 2012 I bought another EEF plate, only to be greatly disappointed, because the scratch pattern was significantly coarser and included a greater number of rogue over-deep scratches. And I had also a disappointing experience with a later DMT hand held 4″ EEF stone as well.
Tried to break in this plate with the Spyderco ceramic and also with the small DMT EEF hand hone, but all to no effect. Something seems to have changed in their manufacturing.
On another note, I have been using a Spyderco UF bench stone for razors and had mixed results, but after much experimenting have found that frequent lapping and the use of WIndex has improved its performance greatly and yields a finer finish than my old Thuringian water stone. Would be interesting if you could look at these fused ceramic stones under the electron microscope because their cutting action appears to be quite different from that of particulate stones.
As an aside, all of the above observations were made under an optical microscope at 40x-80x
Cheers
John
LikeLike
It’s possible that the over-sized diamonds are deliberately added to produce a “toothy” edge (for want of a better term). I’ve observed these on my EEF plate and all four of the EF products I own. This may be preferred for some knives, but it’s not what we want for razors, chisels and plane blades. Certainly the plates can be “broken-in” to remove or smooth those over-sized diamonds, but it surprises me how much wear results in the process.
Sintered alumina (ceramic) stones are equivalent to Arkansas stones, but made of aluminum oxide rather than silicon oxide. In both cases, the preparation of the surface has a dramatic effect on their performance. I am currently working on an article that demonstrates steeling with various rods, including a sintered aluminum oxide.
LikeLike
Hi Todd,
I have observed something similar with Shapton water stones and some of the their purveyors have claimed that an uneven abrasion pattern is both intentional and desirable, so your suggestion of the same being the case with DMTs is plausible – But to me, this just sounds like poor quality control being rationalized and concur with Brent Beach (woodworker) who writes quite a bit on this subject on his website; He prefers coated paper and film abrasives for sharpening his tools.
I too have tried films and papers and the quality of finish obtained was consistently better than from stones, but find that for me they are more trouble than what they are worth because of storage and portability problems.
Be that as it may, for some of the super steels, diamond is the only practical solution, and I get very good edges indeed with my earlier DMT EEF plates followed by stropping on balsa coated with diamond powder.
Looking forward with what you come up on steeling, as there appear to be three distinct and concurrent actions present, namely: Straightening of rolled edges, burnishing (plastic flow) and abrasion.
Keep up the good work, and
Cheers
John
LikeLike
Is it not the future to grow carbides of perfect size inside a steel and use such a plate for honing/steeling? A quick etching with acid would expose them.
If you had to choose a steel plate today what steel would it be?
LikeLike
Interesting idea, but I’m not sure how popular it would be as most metallographic etchants are fairly hazardous.
LikeLiked by 1 person
Todd, what is your opinion of the very best way to prepare a DMT plate for a reasonably uniform surface? Does it make sense to lap a new diamond plate with another to achieve results efficiently/faster while attempting to preserve as much useful life as possible? I’m inclined to believe that a 1:1 ratio of diamond size, i.e. 600grit on 600 grit, or 1200grit on 1200 grit plates would be interesting. Thoughts? Also, just for grins, I wonder if any alternative substrates dressed with fine diamond might offer a suitable method for effectively “breaking-in” a new DMT diamond plate.
On a personal side note: Thank you for all this incredible work. And, your no-nonsense presentation of results. I cannot thank you enough for helping me to quit wasting my time. You deserve a high-place in the sharpening hall of fame. Over time, I believe attribution to your myth busting images will ensure that is so.
Kenny
LikeLike
I definitely wouldn’t use another diamond plate. You only want to hit the rogue diamonds and not remove any nickel. The loose/proud/oversized diamonds are probably best tamed with hard, smooth steel. I’ve always started with a chromium plated screwdriver shank, then just sharpened some high-carbide blades.
LikeLike
In industrial production grinding(high power, calculated feed rates, etc) diamond is used for everything except ferrous metals where cBN is the choice. The reasons are that at the friction temperatures created the diamond softens a fair bit (though still quite hard) and the bigger issue is that the carbon dissolves into the hot iron, rapidly wearing the diamonds.
If one has access to a machine shop with a surface grinder and a lathe, you could level these “consumer grade” [low quality] plates by making a mild steel wheel that fits on the surface grinder and reverse grinding the diamond plates.
Being carbon you could also burn them off with electric discharge machining but that may be tedious unless you had a way to use a flat plate and hit several points at once. The plate would need a precise jig and still need to move laterally as it pitted but choice of current direction can concentrate the energy on the diamond’s side.
A chemical oxidizer could be another option, maybe applied in a thin film over an inert plate and the DMT plate lowered face down on to it. Or similar but electrolysis oxidation rather than a chemical oxidizer?
LikeLike
The key to removing stray oversized diamond pieces is obviously in the manufacturing process !! The manufacturer has to keep certain areas free from cross contamination ! And the media also has to be strained adequately so that there is no oversized diamonds !!
LikeLike
I’m leaning towards believing the large particles are deliberately added, as they are in number of other types of stones.
LikeLike
Todd, thanks for your stunning content 👍
Could you allow me to use 2 SEM photos from that article (commercial purpose), or just sell me that shots?
*Fingers crossed
Yan
LikeLike
Yes Yan, that is fine provided the source is acknowledged.
LikeLike
It’s great! Thank you, Todd!
Could you share with me your contact email, so I can show you how I’ll acknowledge your photos?
LikeLike
scienceofsharp@scienceofsharp.com
LikeLike
Got it. Many thanks)
LikeLike
This is truly great information for the technically minded geek.
I recently purchased a DMT EF (1200, green) Serrated knife sharpener (cone stick), after liking the fine (600) that I purchased last year. I noticed what felt like a few bumps along its length, so I decided to break it in by sliding it along a chrome screwdriver shaft. Then I noticed that it felt and looked like it had no diamonds or texture to it at all. I broke out my cheep USB microscope to see and compare it to several of my other DMT plates and the 600 serrated sharpeners. What I found is what appears like the 1200 separated and my basically unused 1×4 1200 mini-plate both seem to have no diamonds, but all of the others (325 2ea , 600 2 each, extra coarse) have quite obvious large quantities of diamonds on their surface. I can see a few rouge stones that are way too big to be 1200, but elsewhere it looks to be bare metal. I tried cleaning it with Windex, ammonia, but that did not reveal anything. I then thought to try to expose the diamonds by trying to abrade the surface (concrete, Arkansas stone, SIC stone, glass), but nothing seemed to do the trick. As far as I can tell they are either defective or fake. The two 1200g tools do not scratch glass. Thoughts?
LikeLike
There is only a thin layer of diamond-filled nickel on the surface, and it is quite easy to remove with a coarse abrasive. It is also possible that the substrate wasn’t prepared correctly prior to nickel deposition (manufacturing defect) leading to delamination of the nickel. If you can’t scratch glass, I would say you have removed the abrasive coating. As far as I know DMT is fairly generous with replacing defective products.
LikeLike
Great article! Thank you for educating us!
LikeLiked by 1 person